Boyle’s Law (Pressure-Volume Relationship) :

`text(Definition :)` At constant temperature, the pressure of a fixed amount (i.e., number of moles `n`) of gas varies inversely with its volume. This is known as Boyle’s law.
`=>` Mathematically, it can be written as
`p prop 1/V` ( at constant T and n) ...........(1)
`=> p = k_1 1/V` ......................(2)
where `k_1` is the proportionality constant.
● The value of constant `k_1` depends upon the amount of the gas, temperature of the gas and the units in which `p` and `V` are expressed.
● On rearranging equation (2) we obtain
`pV = k_1` .............(3)
● It means that at constant temperature, product of pressure and volume of a fixed amount of gas is constant.
`=>` If a fixed amount of gas at constant temperature `T` occupying volume `V_1` at pressure `p_1` undergoes expansion, so that volume becomes `V_2` and pressure becomes `p_2`, then according to Boyle’s law :
`p_1V_1 = p_2V_2 = ` constant ...........(4)
`=> p_1/p_2 = V_2/V_1` ..............(5)
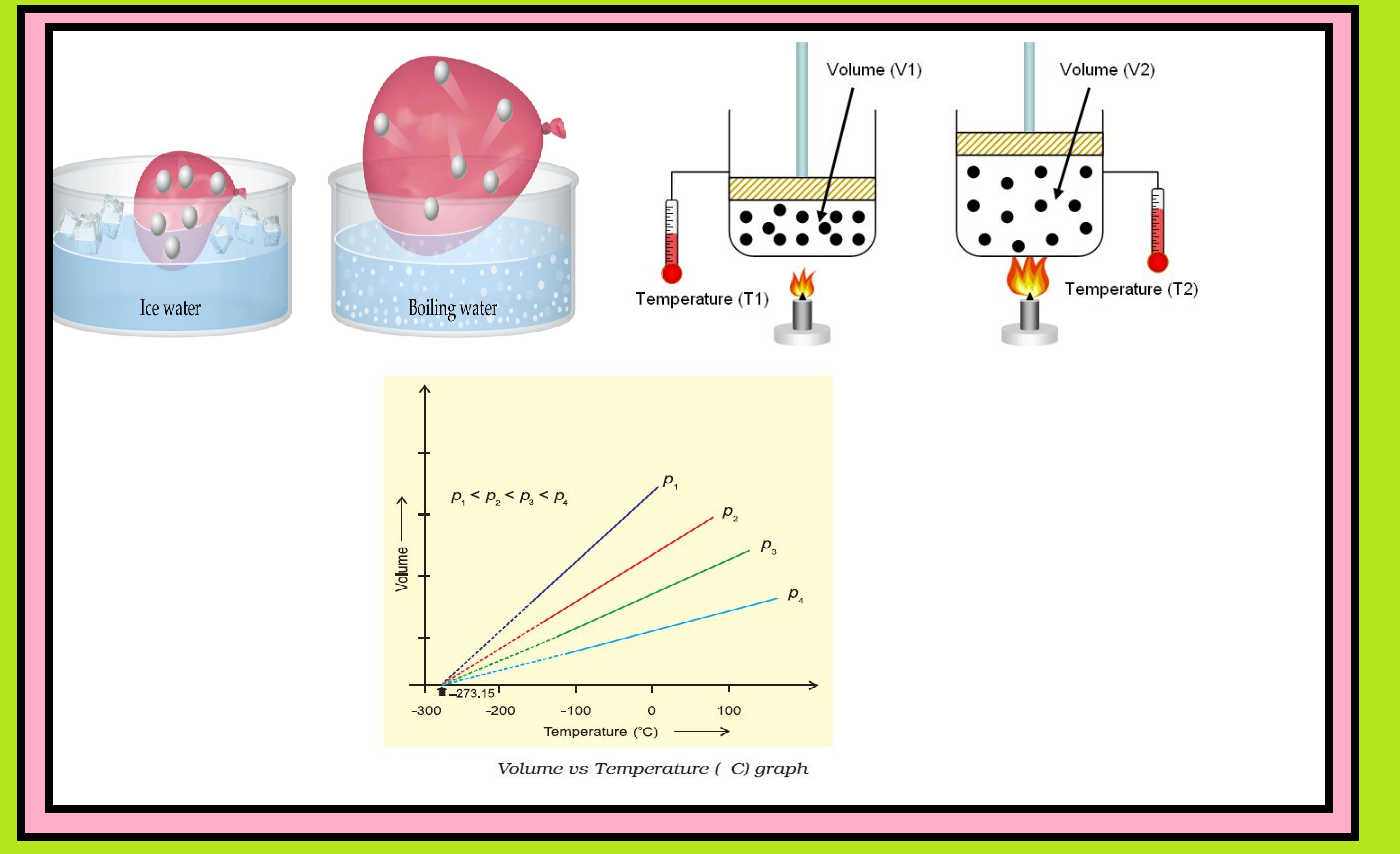
`=>` Figure shows two conventional ways of graphically presenting Boyle’s law.
`=>` Fig. is the graph of equation (3) at different temperatures.
● The value of `k_1` for each curve is different because for a given mass of gas, it varies only with temperature.
● Each curve corresponds to a different constant temperature and is known as an `text(isotherm)` (constant temperature plot).
● Higher curves correspond to higher temperature.
● It should be noted that volume of the gas doubles if pressure is halved.
● Table gives effect of pressure on volume of `0.09` mol of `CO_2` at `300 K`.
`=>` Fig above represents the graph between `p` and `1/V`.
● It is a straight line passing through origin.
● However at high pressures, gases deviate from Boyle’s law and under such conditions a straight line is not obtained in the graph.
`=>` Experiments of Boyle, in a quantitative manner prove that gases are highly compressible because when a given mass of a gas is compressed, the same number of molecules occupy a smaller space.
● This means that gases become denser at high pressure.
● A relationship can be obtained between density and pressure of a gas by using Boyle’s law :
● By definition, density `‘d’` is related to the mass `‘m’` and the volume `‘V’` by the relation `d = m/V`.
● If we put value of `V` in this equation from Boyle’s law equation, we obtain the relationship.
`d = (m/k_1) p equiv k'p`
`=>` This shows that at a constant temperature, pressure is directly proportional to the density of a fixed mass of the gas.
`color{purple}♣ color{Violet} " Just for Curious"`
At higher altitudes, as the atmospheric pressure is low, the air is less dense. As a result, less oxygen is available for breathing. The person feels uneasiness, headache etc. This is called altitude sickness. That is why the mountaineers have to carry oxygen cylinders with them
`=>` Mathematically, it can be written as
`p prop 1/V` ( at constant T and n) ...........(1)
`=> p = k_1 1/V` ......................(2)
where `k_1` is the proportionality constant.
● The value of constant `k_1` depends upon the amount of the gas, temperature of the gas and the units in which `p` and `V` are expressed.
● On rearranging equation (2) we obtain
`pV = k_1` .............(3)
● It means that at constant temperature, product of pressure and volume of a fixed amount of gas is constant.
`=>` If a fixed amount of gas at constant temperature `T` occupying volume `V_1` at pressure `p_1` undergoes expansion, so that volume becomes `V_2` and pressure becomes `p_2`, then according to Boyle’s law :
`p_1V_1 = p_2V_2 = ` constant ...........(4)
`=> p_1/p_2 = V_2/V_1` ..............(5)
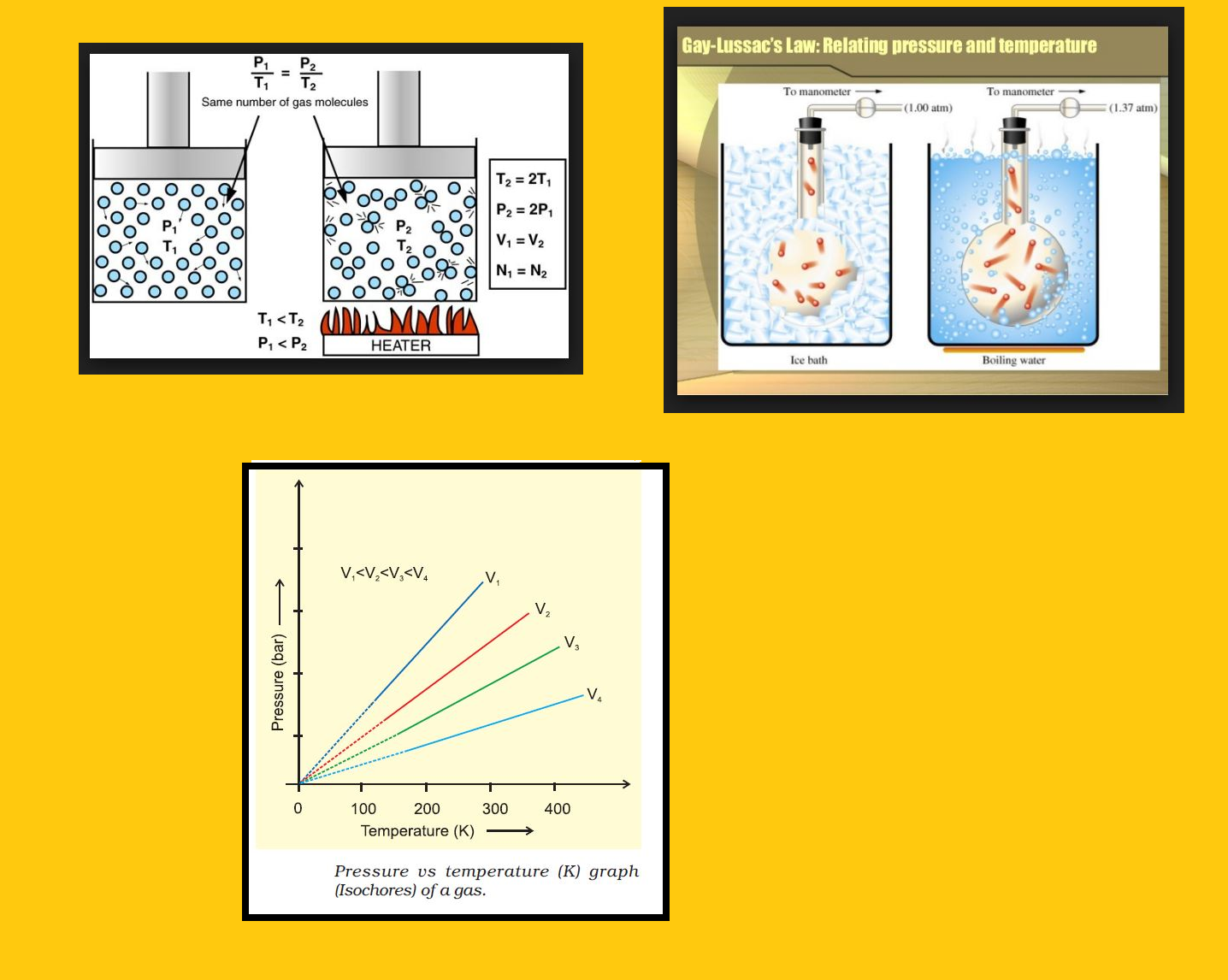
`=>` Figure shows two conventional ways of graphically presenting Boyle’s law.
`=>` Fig. is the graph of equation (3) at different temperatures.
● The value of `k_1` for each curve is different because for a given mass of gas, it varies only with temperature.
● Each curve corresponds to a different constant temperature and is known as an `text(isotherm)` (constant temperature plot).
● Higher curves correspond to higher temperature.
● It should be noted that volume of the gas doubles if pressure is halved.
● Table gives effect of pressure on volume of `0.09` mol of `CO_2` at `300 K`.
`=>` Fig above represents the graph between `p` and `1/V`.
● It is a straight line passing through origin.
● However at high pressures, gases deviate from Boyle’s law and under such conditions a straight line is not obtained in the graph.
`=>` Experiments of Boyle, in a quantitative manner prove that gases are highly compressible because when a given mass of a gas is compressed, the same number of molecules occupy a smaller space.
● This means that gases become denser at high pressure.
● A relationship can be obtained between density and pressure of a gas by using Boyle’s law :
● By definition, density `‘d’` is related to the mass `‘m’` and the volume `‘V’` by the relation `d = m/V`.
● If we put value of `V` in this equation from Boyle’s law equation, we obtain the relationship.
`d = (m/k_1) p equiv k'p`
`=>` This shows that at a constant temperature, pressure is directly proportional to the density of a fixed mass of the gas.
`color{purple}♣ color{Violet} " Just for Curious"`
At higher altitudes, as the atmospheric pressure is low, the air is less dense. As a result, less oxygen is available for breathing. The person feels uneasiness, headache etc. This is called altitude sickness. That is why the mountaineers have to carry oxygen cylinders with them